Pediatric Drug Information Collection Network Development Project

Background
Pediatric drug development targets a wide range of patients, from newborns to adolescents, and therefore, requires a detailed response to each age group in terms of drug formulation and dosage, as well as difficult safety and efficacy evaluations, and also requires pediatric-specific considerations in planning clinical trials and obtaining consent. These requirements place high barriers against the pharmaceutical companies implementing pediatric drug development on their own initiative. In addition, because of the difficulties in conducting clinical trials for pediatric drugs, sufficient data on the efficacy and safety of the drugs cannot be collected, and as a result, pediatric dosage and administration are often not established or specified in the package inserts. Even under such circumstances, healthcare professionals in the medical field use drugs for which pediatric dosage is not clearly stated in the package inserts by reducing the dosage or changing the formulation as needed, but another challenge is that information on their actual usage has not been sufficiently accumulated. Whereas many new drugs are expected to be developed in the future due to innovations in the medical industry, it is necessary to create an environment in which drugs can be used safely and securely for use in children.
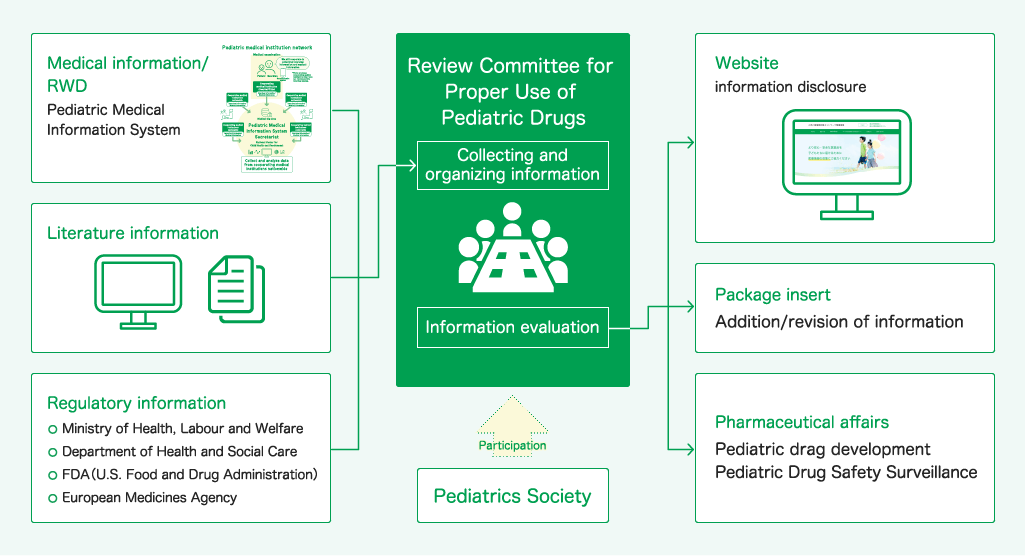
Overview
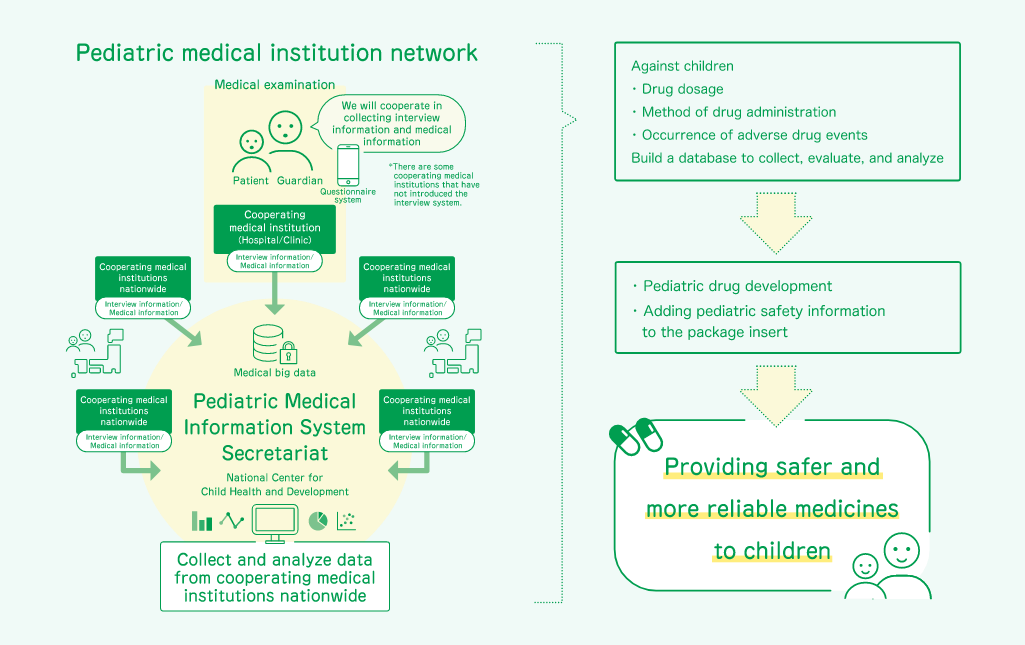
The secretariat of the Pediatric Medical Information Collection System (P-MICS) was established at the National Center for Child Health and Development (NCCHD) as part of the Pediatric Drugs Information Collection Network Development Project, which started in FY2012 as a project subsidized by the Ministry of Health, Labor, and Welfare (MHLW). The secretariat has developed the P-MICS using a network of pediatric medical institutions nationwide as a data source for electronic medical records (EMRs) and medical interview systems, and has established an infrastructure to collect, analyze, and evaluate information on adverse events and dosage of pediatric drugs in the P-MICS database (P-MICS DB).
Objective
The objective of this project is to further improve safety measures for pediatric drugs and to promote the development of pediatric drugs, thereby providing safer and more reliable drugs for children by using the data collected in the P-MICS DB and analyzing and evaluating the actual usage of drugs for children, including dosage and administration methods, as well as information on drug safety, including the occurrence of adverse events.